Following are the main points of Bohr's theory or Bohr's Atomic model.
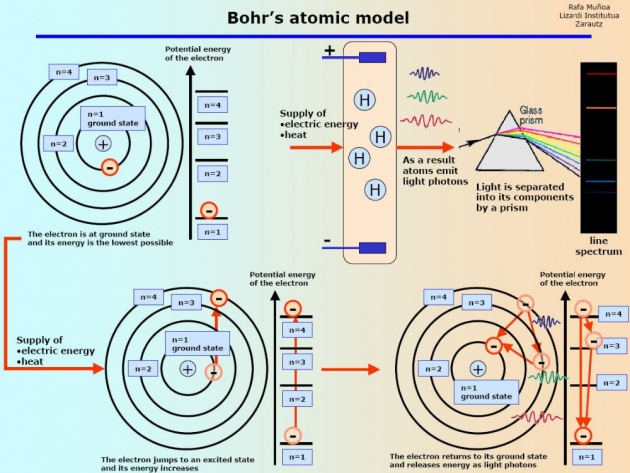
1. It was only for the planetary motion.
2. Bohr calculate the radius of hydrogen atom.
3. Also calculate the Energies of shells.
4.Energy Level Diagrams.
5.It was only for the principal quantum numbers.
6.Only the size of atom was determined by Bohr.
7.It was just for the visible region of the spectrum means only for Balmer Series.
8.Electron revolves around the nucleus in fixed distance.
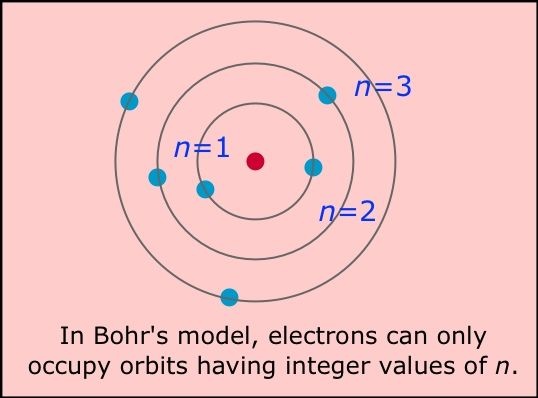
Following are the few drawbacks of Bohr's atomic model.
1.It was just for the single atom.
2.Its only fit with hydrogen atom.
3.Electron revolves around the nucleus in any distance.
4.It does not explain the fine structure.
5.Bohr's theory doesnot follows the Heisenberg's uncertainty principle.