Today, i am going to discuss the method are you can say that the procedure by using which we can calculate the percentage of antimony in lead. The procedure include simple steps.
First we have to take one gram sample of lead in which we have to calculate the percentage of antimony. Then take 25 ml sulfuric acid in a flask and put it on heater.

Now, put one gram of lead sample in it and allow it to boil until all the pieces of lead melt. After boiling remove the flask from the heater and allow it to cool.

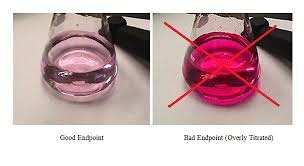
After cooling add 100 ml distilled water in it. Now add 25 ml hydrochloric acid. Then proceed to the next step which is titration. We use methyl orange as an indicator.

Then titrated solution by potassium per magnate. Titration remain continues until the orange color becomes disappear. Now, take the reading of potassium per magnate in ml which is use from starting point to the point when the orange color disappear.
Now, the reading which is obtain is multiply with 0.006088 and then multiply it to 100 and divided by the weight of sample which is 1 gram. By using this procedure we can easily calculate the percentage of antimony in lead.



