Sulfuric acid was first prepared by a Muslim scientist in 9th century. They prepared by the distillation of sulfuric acid by using contact process. In this process, sulfur dioxide and is passing over a hated catalyst. By doing this sulfur dioxide is converted into sulfur try oxide.
This sulfur try oxide is dissolved in sulfuric acid to form paste. When the paste is prepared then it react with water to form sulfuric acid. The physical properties of sulfuric acid are that, the color of concentrated sulfuric acid is brown but the dilute sulfuric acid is colorless. Its specific gravity is 1.84 .

It is odorless and very corrosive to the skin. Concentrated sulfuric acid is very dangerous for human beings. The boiling point of sulfuric acid is 348 degree centigrade and freezing point is 10.5 degree centigrade. Concentrated sulfuric acid do not conduct electricity but when they are dilute, then they are good conductor of heat and electricity.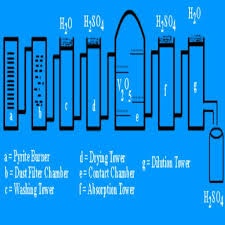
Chemical properties of sulfuric acid are that, it react with sodium hydroxide to from sodium hydrogen sulphate and sodium sulphate. When sulfuric acid react with carbonates and bicarbonates to from salt , water and carbon dioxide.
Sulfuric acid is used in the manufacturing of hydrochloric acid, nitric acid and phosphoric acid. They are used for the preparation of dyes, explosives and fertilizers.sulfuric acid is used in medicine. It is used drying agent and used in refining, dying and paper industries.



