An instrument which measures the exact masses of different isotopes of an element is called mass spectrometer and this technique is called mass spectrometry. First of all Aston’s mass spectrometer was used to identify the isotopes of an element. The Dempster’s Mass spectrometer is used to identify isotops of solid elements. The principle of mass spectrometer is that the positive ion of each isotope has its own (m/e) value the isotopes are separated on the basis of their mass to charge ratio (m/e). “Construction and Working” is given as,the substance (isotopic element) under analysis is converted into vapours. The Pressure of these vapours is kept very low. These vapours enter into ionization clamber. Here fast moving electrons collide with vapours. Thus vapours are ionized. Different isotopes produce positive ions with different (m/e) values. These positive ions are accelerated by applying potential difference of 500----2000 volts to the perforated negative grid. Then positive ions are passed through a strong magnetic field. This field separates the ions on the basis of their (m/e) values. A simple diagram of mass spectrometer is shown below.
After passing through the magnetic field the ions move in a circular path. Finally these ions fall on an electrometer (ion collector)in the form of groups. The (m/e) of ions is determined by following formula.Where H is strength of magnetic field, E is strength of electric field, and r is the radius of circular path.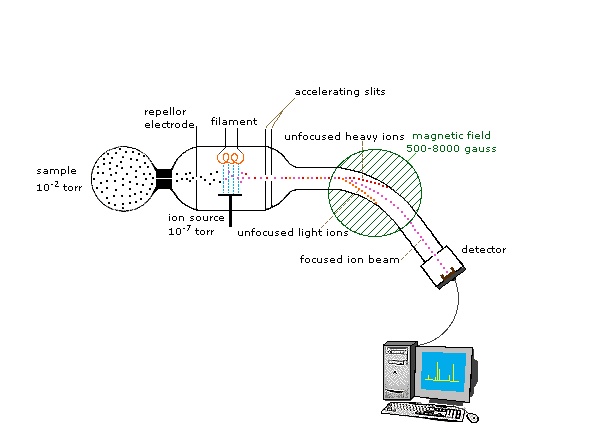
The electrometer produces (develops) electric current. The strength of electric current gives relative abundance of ions weigth definite (m/e) value. Thus relative abundance of the isotopes is determined. This experiment is repeated for C-12 isotope. By comparing two currents we can measure exact mass number of isotope. In modern spectrographs, the electric current is amplified and fed to the recorder. The recorder makes a graph between relative abundance and mass number of isotopes. Such a graph for three isotopes of Neon is shown in figure.