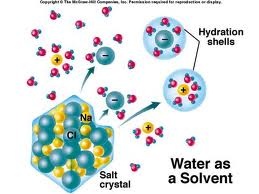
Today, we are going to talk about solvents. As we know solvent is that which can dissolve solute. Water is known as a universal solvent because it can dissolve large number of organic and inorganic substance without giving any temperature. Water is an aqueous solvent.

Now, we are going to talk about non aqueous solvent. Other than water if we use any other solvent for dissolving solute is known as non a non aqueous solvent. For example, hydrogen fluoride is non aqueous solvent. It is the solvent which have maximum water like properties among all the non aqueous solvent.
It can dissolve large number of organic and inorganic compounds but it have one disadvantage which is that it has poisonous characters. Hydrogen fluoride self dissociate to give Fluorine ion. From theory of solvent any solvent which can give us fluorine ion will be act as a strong acid.

Acids such as nitric acid and sulfuric acid which act as a strong acid in aqueous medium, when we change the medium then the same acids will be act as base. So, it is clear that by changing the nature of solvent the nature of solute will also be changed without increase or decrease in temperature..



