The American physicist R.A. Millikan measured the elementary charge e. The oil droplets are introduced into the chamber by an atomizer, some of them become charged either positive or negative, in the process. Consider a drop that finds its way through a small hole in the chamber which is under the first chamber. Let us assume that the charge carried by the droplet is q which we take to be negative.
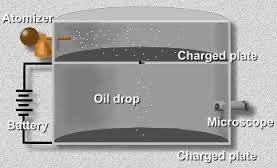
If there is no electric field, two forces act on the drop, its weight mg and an upward directed viscous drag force, whose magnitude is proportional to the speed of the falling droplet. The drop quickly comes to a constant terminal speed v at which these two forces are just balanced.
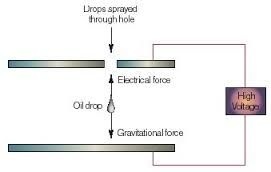
A downward electric force is now set up in the chamber by connecting the battery. A third force qE now acts on the drop. Since q is negative so this force acts upwards and we assume that the drop now drifts upwards at a new terminal speed v'. In each case the drag force points in the direction opposite to that in which the drop is moving and its magnitude is proportional to the speed of the drop.
The charge q on the drop can be found from the measurements of v and v' .
Millikan found that the values of q were all consistant with the relation:
q = ne n = ...., -2, -1, 0, 1, 2, ....
in which e is the elementary charge, whose value is 1.60*10^-19 C. Millikan's experiment is a convincing proof that the charge is quantized.
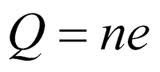
Millikan was awarded the 1923 Nobel Prize in physics in part for this work. Modern measurements of elementary charge rely on a variety of of the interlocking experiments, all more precise than the pioneering experiment of Millikan.