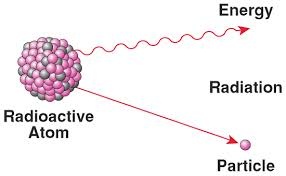
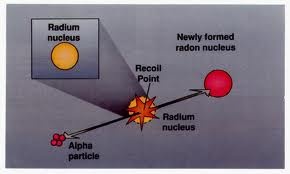
In this article i will talk about the nuclear radioactivity elements. It is the phenomena of a emission of radiation from nuclear of certain items. The properties of that element are alpha beta and gamma rays. Alpha rays have double charge as compared to the proton.
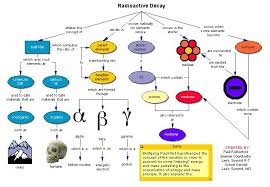
The ionization power is greater then the other element. They can produce Florence. After traveling a little they are absorbed in the air and the beta rays are highly radioactivity rays and their speed in a really equal to the velocity of light and can travel at the speed of light.
They have great potential power. They affect photographic plates and cause ionization of gas and air molecules. The third form of radioactivity elements are the gamma rays that are very powerful in energy as compared to other element.
Gamma rays also travel like the speed light and their travels equal to the alpha and beta rays .Being naturally these rays pass straight through the electromagnetic field. They cause ionization of gas or air molecules. As we know that this radioactivity elements have a very short life because they are destroy in a few seconds.
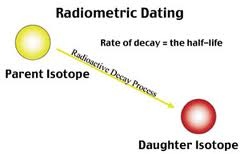
So that is why this is called half life element .Half life of an element mean the time in which one of given weight is disintegrates. Example of half life element are the Protactinium and a if we take a compound of Na that is 100 kg and place in a box and leave for a day. The next day their mass are decrease and the left compound are the only 50 kg.

These rays are very useful and use in a different purposes in the laboratory or industrial field. these rays are use in the photography, x-rays and other difficult operations like laser operation ,all done by these rays. The drawback of this rays are that they are very dangerous for human body if they are fall on our body they can destroy completely and burn our body easily in a few second .