Secondary Structure of Protein:-
The discussions of the primary structure of polypeptides give the impression that peptide chains are quite straight and extended. But X-rays diffraction experiments on peptide and proteins crystals have shown the polypeptide chain tend to twist or coil upon themselves in characteristic manner. The poly peptide is fold into the specific structure held together by H bonds. The regular arrangement of amino acid in segment of polypeptide chain in which each amino acid is spatially related to its neighbor in the same way is called the secondary structure of proteins. It may take one form from α- Helix or β- pleated sheet.
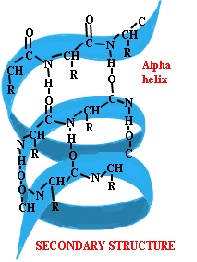
α-Helix:-
It is clockwise, rod like spiral and is formed by intra chain H bonding between the C=O group of the each amino acid and the –NH2 group of the amino acid that is situated four amino acid residues ahead in the linear sequence. The R group of each amino acid residue faces outward.
Because the proteins has only L amino acids, therefore the helix is coiled in a right handed direction which is the more stable form and is the one seen in α-Helix. Each turn of the helix contain 3.6 amino acid residues and each successive turn is 0.5 nm above the turn below it. The α helix shows a bend when proline is reached. This is because the peptide bond nitrogen of this amino acid lacks the hydrogen atom and therefore a H bond cannot result. Proline is however be accommodated within the first turn of an α helix. Glycine being a small sized amino acid also produces such a bend.
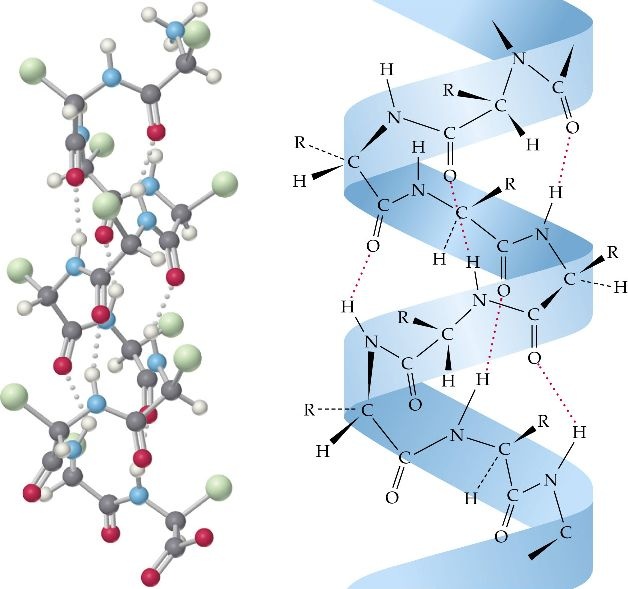
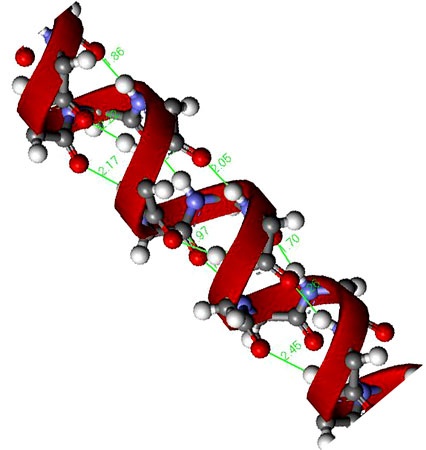
The helical structure gives stability to the peptide molecule because each peptide bond participates in H bonding. The H bond are individually weak but collectively are the major force stabilizing the helix. The other main force helping to maintain the helix is hydrophobic interaction. Proteins containing α helix show great strength and elasticity and can be easily stretched because they are in the form of a tight coil.
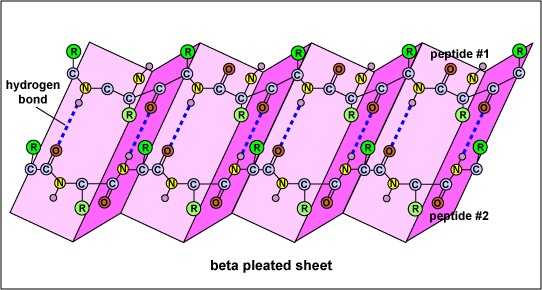
Β- Pleated Sheet (pleated = folded on itself)
In this case the proteins of polypeptide chain containing 5 to 10 amino acid residue from different primary structural regions line up side by side just as sheet of cloth can be folded again and again; H bond exist between these peptide strands running closer to each other. The peptide stands forming Β- Pleated sheets may run in same direction or in opposite direction. Proteins containing this structure are inelastic because the H bond are at right angle to the direction of stretching and simply hold the bundles of adjacent portion of peptide chain together.