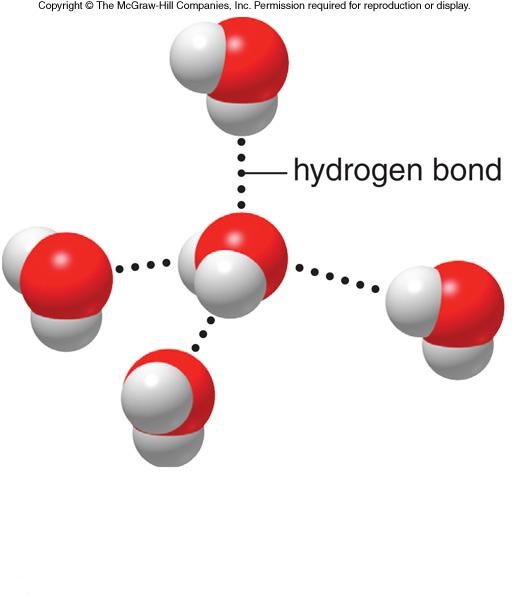
The structure of water is tetrahedral.The two corners of a tetrahedron are occupied by two H-atoms and other two corner are occupied by two lone pairs of electrons of oxygen. Due to two lane pairs of electrons, each oxygen of water molecule can form two H-bonds with hydrogen atoms of two neighbouring water molecules.
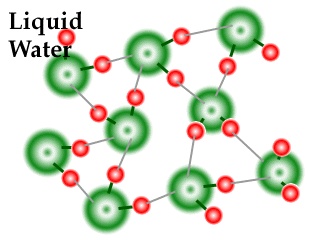
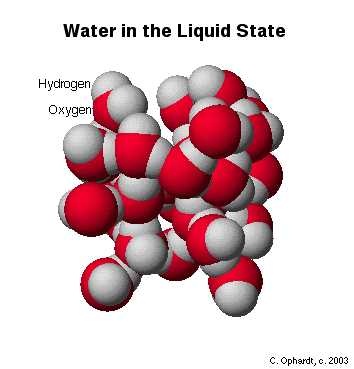
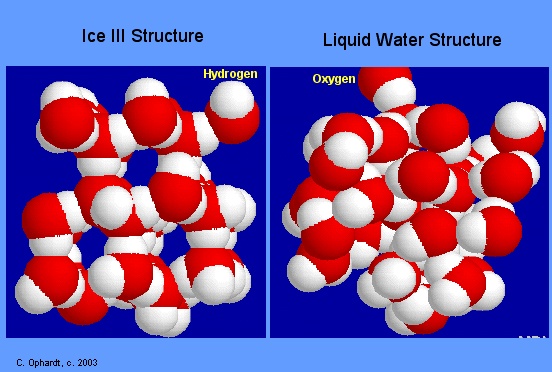
In liquid state water molecules are mobile. So H-bonds are broken and reformed again and again. The strength of hydrogen bond is always less then as compare to covalent bond. The structure of liquid water is shown below.
During ice formation the water molecules become more and more regular. This regularity is present throughout the structure. In this regular structure of ice empty spaces are created. Thus ice occupies 9% more space than the liquid water. So ice has less desity and it floats on water. The structure of ice is just like that of diamond.

In diamond each carbon atom is in the centre of tetrahedron and in ice each oxygen atom is in the centre of tetrahedron. The structure of ice is shown in Figure. During winter, the temperature falls down.Water has maximum density. This water goes to the bottom of pond or lake. But temperature of surface water falls more and more and it freezes into ice.
This ice floats on the surface. Because ice is an insulator of heat, so it prevents underneath water from freezing. Therefore fish, plants and other aquatic animals survive under the blanket of ice.