The word "Matter" mean "The solid particles". The solid particles can be converted into another form. Actually matter is made up of molecules and molecules are made up of atoms. When atoms combines, then molecule is formed and when different molecules combines then matter is obtained. The examples of matters are doors, windows, chair etc. So, matter is defined as "Anything having mass and some space in it molecules, then this is called matter". Matter have three types namely Solid, Liquid and Gas.
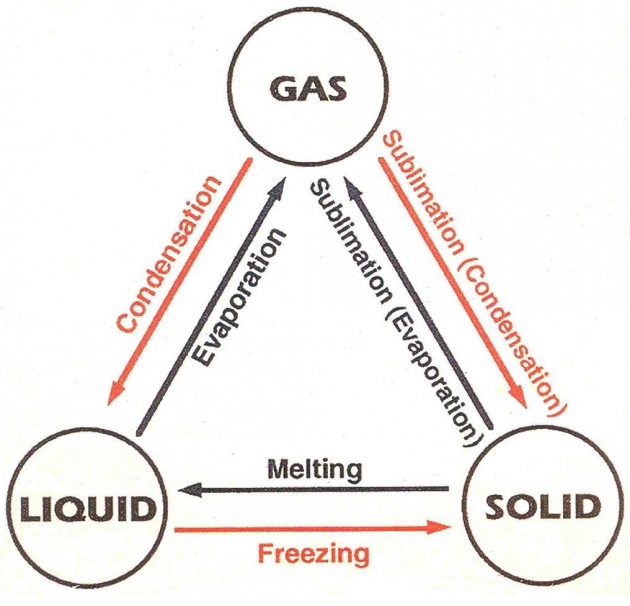
The first type of matter is solid. The example of solids are table, wooden scale ice, chalk etc. In these examples, you will notice that all are solids because in solids, the molecules of solid are closely packed. They have no free space. So, it has proper shape. If the intermolecular forces are not strong, then we cannot see these things.

The 2nd type is liquid. When we heat the solid like ice, they will convert into another form which is liquid. When we heat up the ice, the ice melt and changes into liquid. The molecules of liquid are free move everywhere in container. They have proper shape. The molecules of liquid are not closely packed. The examples of liquid are juices, cold drinks, mixtures of different substances etc.

When we heated liquid, the liquid boiled and converted into another form which is called Gas. The gas molecules are free to move everywhere in the atmosphere. They have no proper shape and no proper volume. The examples are carbon dioxide, oxygen, hydrogen gas etc.

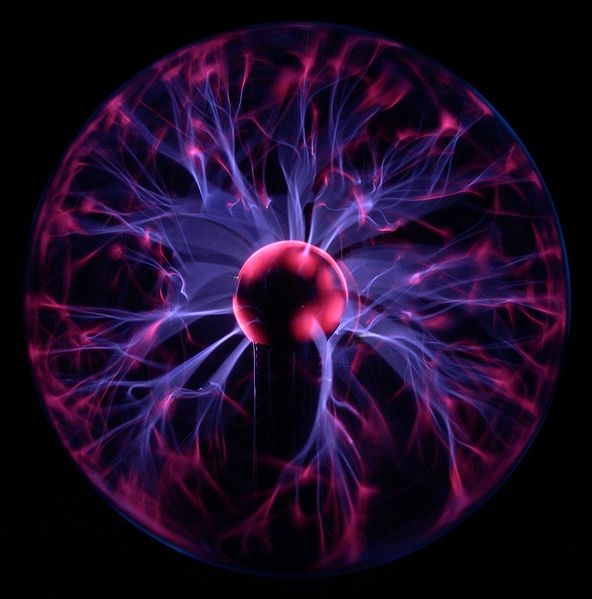
One more type of matter is plasma which is yet not taken in its type. Plasma is a liquid type matter. It exit in very high temperature. So, it cannot found on earth. Plasma is present in sun because of high temperature.It has also free electrons and free atoms.It has also a proper shaped. The sun is in plasma state. Plasma is a fluid type liquid which is required very high temperature, which is not possible on earth.