بسم الله الرحمن الرحيم
Today I write about the Modern Periodic Table in I can showing the function of Modern Periodic Table in detail
Basis of Modern Periodic:
The modern periodic table is based on the atomic number of the elements rather than their atom masses. In other words it is based on the modern periodic law
Statement of the Modern Periodic:
The physical and chemical properties of the elements are the periodic function of their atomic numbers
Classification of the Modern Periodic:
The elements are arranged in order of their increasing atomic number in which the elements have similar properties and, valence electronic confutation is repeated at regular intervals.
Modern form of periodic table:
The modern periodic table in the form of periods and groups
Periods:
Statement of the period:
The Horizontal row of the elements in the periodic table is known as the Periods
Numbers of the period:
There are seven period (Horizontal row) the long form of the periodic table .First period contains two elements (hydrogen and helium) this is the shortest period of periodic table
. The second period and third are each contain eight representative elements and they are called stort period in periodic table.
The fourth and fifth periods have contains 18 elements each: in which the eight elements are also each; in which beside the eight representative additional sets of 10 elements called, transition are also present. In period fourth and fifth transition elements range from Sc to Zn and Y to Cd respectively
The sixth period is a long period in the periodic table. It has been contains 32 elements, eight representative and ten transition elements and fourteen rare earth are lanthanides place below the periodic table.
The seventh period is still incomplete .Actinides series containing 14 elements is taken out form seven period placed separate at the bottom of the table beneath lanthanides series.
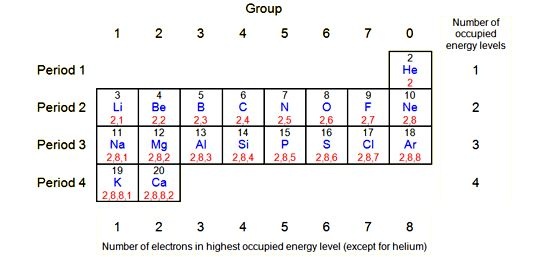
Groups:
Characteristics:
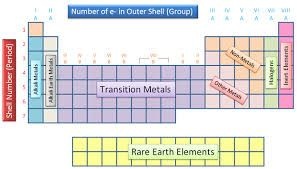
Groups are run top to bottom and containing elements with the same number of electrons in their outer most energy levels. There are eight columns (groups) in the periodic table
Numbers of the Group:
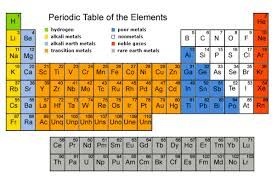
Several of the groups are given special names: the members of Group -1 are called the “alkali metals”, group -2 contains “alkaline earth metals” , groups -6 the Chalcogens group , group – 7 , the halogens and group -8 the noble gases or inert gases
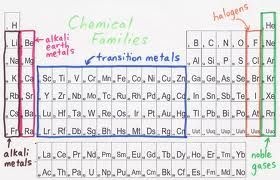
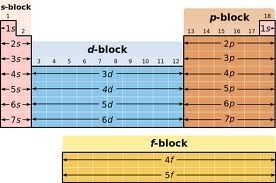
Blocks in the periodic table:
The groups 1 and group 2 elements are called s-Block elements as these elements have s- orbital involved in bond formation with other elements similarly the elements group 4 – 8 are called p – Block elements are called representative elements . The transition elements are called the d- block elements while the lanthanide and actinide series are called the f – block elements
Blog writer
Muhammad Qasim



