The symbol of boron is B . They are metals and have a deficiency of electrons. The length of boron-boron compound is shorter and the halides of boron are monomeric.

The physical properties of boron are that, its atomic number is 5 and atomic weight is 10.82. The melting point of boron is 2300 and boiling point is 2550.The ionization potential of boron is complex .

Boron exist in the form of some compounds that occurs in the deserts of California and India. Chemical properties of boron are that when it reacts with nitric acid to form nitrogen dioxide .When boron react with sulfuric acid as a result sulfur dioxide will be obtained.

When boron react with sodium hydroxide than hydrogen gas is produced. Boron react with carbon dioxide and silicon dioxide to obtain carbon and silicon in solids form.Boron react with chlorine at a very high temperature of 410 degree centigrade to form barium chloride .

when it reacts with iodine at a temperature of 1250 degree centigrade to form barium iodide. It is used as laboratory reagent and it is also used for the preparation of different products .
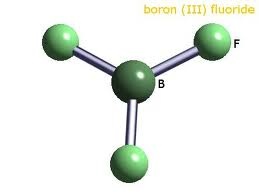
In some factories boron also prepared by reacting barium chloride with hydrogen as a result hydrochloric acid and boron will be obtained.



