It seems very safe to me to be surrounded by green growing things and water.
-Barbara Kingsolver-
Water is one of the most important chemicals known. Without it neither animal nor plant life would exist. Humans can live for a few weeks without foods but only for a few days without water. Almost three-fourths of the Earth's surface is covered by water. Rain, snow, sleet, hail, fog, and dew are manifestations of the water vapor present in the air.

In the figure is a patient's leg showing pitting edema. (Image credits)
Water is present on the solid part of the Earth's surface as lakes, streams, waterfalls, and glaciers. The human body is approximately 50 percent water. Water is essential in the process of digestion, circulation, elimination, and the regulation of the body temperature. Indeed, every activity of every cell in the body take a place in a water environment. Normally, in the body, water intake equals water as *edema results. If water intake is less than output, dehydration occurs. It is important as a solvent. Many substances dissolve in water, sugar, salt, and alcohol are examples.
Courtesy of the video: Natural Ways via Youtube
Physical Properties of Water
Pure water is colorless, odorless, and tasteless. Tap water owes its taste to dissolve gases and minerals. Large bodies of the water such ass lakes and oceans appear blue owing to the presence of finely divided solid material and also to the reflection of the sky.
When water at room temperature is cooled, its volume contracts. However, water is an unusual liquid in that, after it is cooled to 4 degrees Celsius, further cooling causes expansion in volume. When water freezes at 0 degrees Celsius, it expands even more, increasing in volume by almost 10 percent and decreasing its density. These changes on freezing explain why ice floats and why pipes may burst when the water in them freezes. Expansion of water during frostbite explains the damage to the cells.
The density of water changes with temperature. At 4 degrees Celsius water has its smallest volume and its maximum density, 1.00 mL regardless of temperature, since the variation in density between 0 degrees and 100 degrees Celsius is a quiet small.

Image credits
Pure water boils at 100 degrees Celsius at 1 atm pressure. At lower pressures it boils at a lower temperature. In certain mountainous localities, water boils at 80 degrees Celsius because of the lower pressure. If more heat is applied to the water, it merely boils faster. The boiling point does not increase. When water boils at 80 degrees Celsius, there may not be sufficient heat to cook food. In this case, the food must be heated in a pressure cooker. This device increases pressure and so increases the boiling point of the water. The same principle holds in the autoclave, where the increased pressure raises the boiling point.
For water to evaporate, a certain amount of heat is necessary. The amount of heat, called the heat of vaporization, is 540 cal/g. When water is placed on the skin, the heat it needs to make in evaporate comes from that skin. Therefore, the skin loses heat and so is cooled. The evaporation of perspiration also cools for the skin.
For ice to melt, the amount of heat required is 80 cal/g. When an ice packed is placed on the skin, the heat needed to melt the ice comes from the body, thus lowering the temperature of the body.
Effects on Human Civilization
Civilization has historically flourished around rivers and major waterways; Mesopotamia, the so-called cradle of civilization, was situated between the major rivers Tigris and Euphrates; the ancient society of the Egyptians depended entirely upon the Nile. Rome was also founded on the banks of the Italian river Tiber. Large metropolises like Rotterdam, London, Montreal, Paris, New York city, Buenos Aires, Shanghai, Tokyo, Chicago, and Hongkong owe their success in part to their easy accessibilty via water and the resultant expansion of trade. Islands with safe water ports, like Singapore, have flourished for the same reason. In places such as North Africa and Middle East, where water is more scarce, access to clean water was and is a major factor in human development.
-Read more on this source-
Reference: http://chemistry.elmhurst.edu/vchembook/104Aphysprop.html
Impurities Present in the Water
Natural water contains many dissolved and suspended materials. rainwater contains dissolved gases- oxygen, nitrogen, and carbon dioxide- plus air pollutants, suspended gas particles, and other particular matter. Groundwater contains minerals dissolved from the soil through which the water has passed. It also contains some suspended materials. Seawater contains over 3.5 percent dissolved matter, the principal compound being sodium chloride. Both seawater and groundwater also contain dissolved and undissolved pollutants.

Image credits
Lake water or river water may appear clear when a glass full of it is help up to the light, or it may at first contain suspended clay or mud, which tends to settle slowly, leaving what appears to be pure water. However, either of these "clear" waters may contain bacteria and other microorganisms that can be quite harmful to the body. Their destruction or removal is necessary for the proper purification of water. Water can be purified with several processes. The most common are distillation, boiling, filtration, and aeration.
Distillation
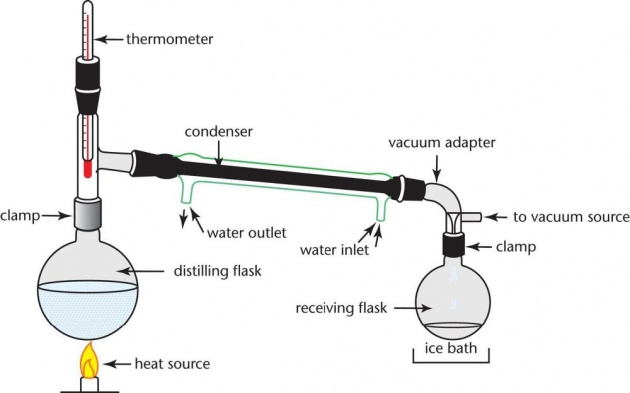
Image credits
Distillation is a process of converting water to steam and then changing the steam back to the water again. In the laboratory, a "still" is used to prepare distilled water. In the still, impure water is placed in the flask at the left and then heated to boiling. As the water boils and changes into steam, it passes into condenser, which consists of a long glass tube surrounded by another glass tube through which cold water runs. The cold water, by absorbing heat, causes the steam to condense back into liquid water, which then runs out of the end of the tube into the receiving vessel at the right. The suspended and dissolved solids (including bacteria) that were present in the impure water remain behind in the flask. They do not pass over into the condenser with the steam. The dissolved gases originally present in the impure water, however, do pass over with the steam. The usual practice is to discard the first few milliliters of distilled water coming from the condenser, since they contain most of the dissolved gases.
Although distilled water is a pure water, it is too expensive and the process too slow for large-scale use. The principle used of distilled water in the hospital in in the preparation of sterile solutions.
Boiling

Image credits
Groundwater or contaminated water can usually be made safe for drinking it for at least 15 minutes. The boiling does not remove the dissolved impurities, but it does kill any bacteria that might be present. Freshly boiled water has a flat taste because of the loss of dissolved gases. The taste may be brought back to normal by pouring the water back and forth from one clean vessel to another. This process, called aeration, allows air to dissolve in the water again.
Sedimentation and Filtration
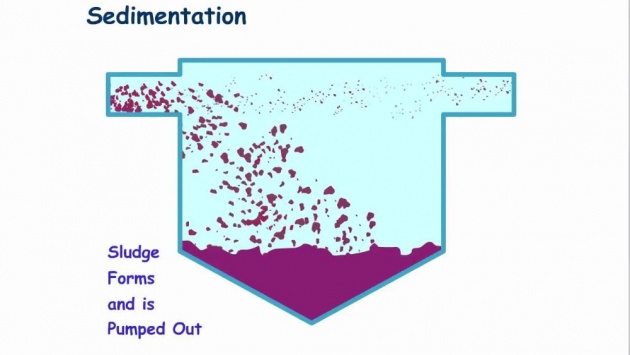
Image credits
For a large-scale use, water is first allowed to stand in large reservoirs where most of the suspended dirt, clay, and mud settle out. This process is called sedimentation. However, sedimentation is very slow process. Put some finely divided clay in a graduated cylinder full of water, shake it, and see how slowly the clay settles out. Commercially, a mixture of aluminum sulfate and lime is added to the water. These two chemicals combine to form aluminum hydroxide which precipitates as a gelatinous (sticky) substance. As the sticky aluminum hydroxide settles out, it carries down with it most of the suspended material. The main advantage of this material is that it settles much more rapidly than does the suspended material by itself and so increases the rate of sedimentation.
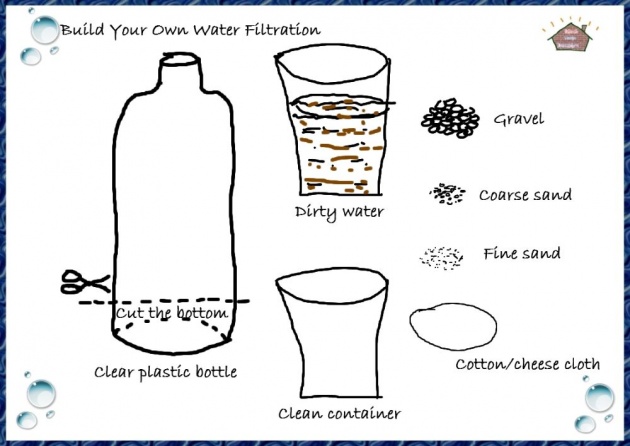
Image credits
After sedimentation, the water is filtered through several beds of sand and gravel to move the rest of the suspended material. The water then is essentially free of suspended material. However, this process doe not remove the dissolved material of or much of the bacteria originally present. The water must then be treated with chlorine to kill the bacteria before it is fit to drink.
Aeration

Image credits
Water can be purified by exposing it to air for a considerable period of time. The oxygen in the air dissolves in water and destroys the bacteria by the process of oxidation. The oxygen also oxidizes the dissolved organic material in the water so that the bacteria have no source of food. However, this process is slow and expensive because of the length of time involved in exposing water to the air. Commercially, aeration is accomplished by spraying filtered chlorinated water into the air. This additional process also removed objectionable odors from the water.
References:
- https://aosts.com/what-are-impurities-in-water-common/
- https://www.wildernessawareness.org/articles/how-purify-water-water-purification-process
- https://en.wikipedia.org/wiki/Water_purification
Hard and Soft Water

When hair is shampooed in hard water, curd clings to the hair strands, dulling their natural luster and interfering with their ability to reflect light. The hair strands at top are stringy and not clean because of the clinging hard water curd. Those at bottom, washed in soft water, are radiant and clean. (Image credits)
When a small amount of soap is added to a soft water, it forms copious suds. When a small amount of soap is added to hard water, it forms a precipitate or scum and no lather. What is the difference between soft water and hard water? Hard water contains dissolved compounds of calcium and magnesium. Soft water may contains other dissolved compounds, but these compounds do not cause hardness. The calcium and magnesium compounds (which cause the hardness) react with soap to precipitate, thus removing the soap from the water. More and more soap must be added until all the hardness-causing compounds are removed. Only then will the soap cause a leather.
The precipitated soap adheres to washed materials, making them rough and irritated to tender skin, or to washed hair, making it sticky and gummy. Food cooked in hard water is likely to be tougher than that cooked in soft water because of the presence of additional minerals. When hard water is boiled, some of the salts from a deposit on the inside of the container in which is heated. Look inside an old teakettle at home and see the "boiler scale." Hard water must never be used to sterilize surgical instruments because the precipitated salts will dull the cutting edges. (If iron compounds are present in the water, they will also cause hardness.)
Detergents have replaced soaps for washing clothes because they do not precipitate in a hard water.
Home Methods for Water Softening
Temporary hardness is hardness that can be removed by boiling. It is caused by the bicarbonates of calcium and magnesium. Boiling converts these to insoluble carbonates and carbon dioxide gas, thus removing part of hardness.
Other soluble compounds of magnesium and calcium cause permanent hardness. Permanent hardness is not affected by boiling.
Ammonia (ammonium hydroxide) is frequently used to soften water used in washing clothes and windows because it precipitates all the ions that cause temporary hardness and some of those that cause permanent hardness. Borax, sodium tetraborate, has been used in home as a laundry water softener. Washing soda frequently used as water softener, removes both temporary and permanent hardness from the water. Trisodium phosphate or TSP, which is another home laundry water softener, has an action similar to that of washing soda. However, TSP no longer recommended for laundry use because of the effects of phosphates on our lakes and streams.
Hard water refers to the concentration of certain substances like calcium and lime in your water. If your water contains calcium, a quick boil may remove its funny taste. Other contaminants can be removed with filters. For better water throughout your household, consider installing an ion exchange system. Laundry water, on the other hand, can be softened with baking soda and vinegar.
-Read more on this source-
Reference: https://www.freedrinkingwater.com/water-education/quality-water-hard.htm
Fluoridation of Water
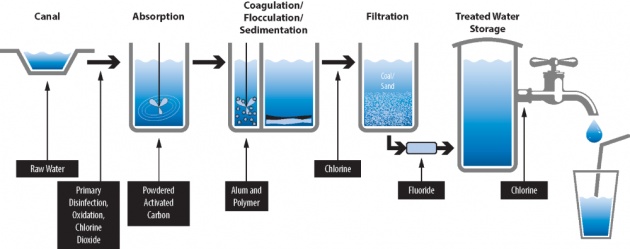
Image credits
Approximately 1 ppm of fluoride ion added to drinking water in the form of NaF reduces the incidence of dental caries. One of the major constituents of bones and teeth is hydroxyapatite. The presence of hydroxide ion makes this substance susceptible to attack by acidic substances by such as soft drinks with low PH. Replacement of hydroxide ion with a fluoride makes teeth more resistant to acid attack and hence more resistant to decay.
Fluorides are also used in toothpastes and gels in the form of stannous fluoride.
Water as a Moderator
Many nuclear reactors use water as a moderator, a substance that slows down the neutrons. Why slow down neutrons? Because slower-moving neutrons can react with 235 U more effectively than faster-moving neutrons.
Reference: https://en.wikipedia.org/wiki/Water_fluoridation
Water Pollution
What is a polluted water? Strictly speaking, it is any water that is not pure. However, tap water contains many dissolved and suspended substances. It is not pure, yet it is not called polluted water. Any substance that prevents or prohibits the normal use of water is termed pollutant. The signs of polluted water are usually quite obvious-oil and dead fish floating on the surface of a body of water or deposited along the shores, a bad taste to drinking water, a foul odor along the waterfront, unchecked growth of aquatic weeds along the shore, or tainted fish that cannot be eaten. Water pollutants can be classified into several categories.
- Oxygen-demanding wastes. Dissolved oxygen is required for both plant and animal life in the body of water. Anything that tends to decrease the supply of this vital element endangers the survival of the life forms. Oxygen-demanding wastes are acted upon by bacteria in the presence of oxygen, thus leading to a depletion of the dissolved oxygen. Oxygen demanding pollutants include sewage and wastes from papermills, food processing plants, and other industrial processes that discharge organic materials into the water.
- Disease-causing agents. Among the diseases that can be caused by pathogenic microorganisms present in polluted water are typhoid fever, cholera, infectious hepatitis, and poliomyelitis.
- Radioactive material. Low-level radioactive wastes from nuclear power plants are sealed and in concrete and buried underground. High-level radioactive wastes are initially stored as liquids in charge underground in large underground tanks and later converted into solid from for burial in concrete. In either case, leakage can lead to pollution of nearby water supplies.
- Heat. Although heat is not normally considered a pollutant of waterways, it does have detrimental effect on the amount of dissolved oxygen. Thermal pollution results when water is used as a coolant for industrial plants and nuclear reactors and then returned to its source. In addition to decreasing the amount of dissolved oxygen, thermal pollution also causes an increase in the rate of chemical reactions. The metabolic processes of fish and microorganisms are speeded up, increasing their need for oxygen, at a time when the supply of oxygen is diminishing. Higher water temperatures can also be fatal to certain forms of marine life.
- Plant nutrients. Nutrients stimulate the growth of aquatic plants. This may lead to lower levels of dissolved oxygen. It may also lead to disagreeable odors when the large amount of plant materials decay.s. Excessive plant growth is often unsightly and interferes with recreational use of water. Excess phosphorus in sewage comes from phosphate detergents and is one cause of this type of pollution.
- Synthetic organic chemicals. In this category of pollutants are such substances as surfactants in detergents, pesticides, plastics, and food additives.
- Inorganic chemicals and minerals. These pollutants come from industrial wastes as well as from runoff water from urban areas. One example of such pollutant is mercury. It was once believed that metallic mercury was inert and settled to the bottom of a lake. It is now known that anaerobic bacteria in bottom muds are capable of converting this mercury into compounds that are poisonous. Another pollutant in this category is sulfuric acid, which is formed by the reaction of sulfur-containing ores with water and oxygen in the air. Salt is also a pollutant, which can occur when brine from oil wells is released into freshwater.
One of the examples of polluted water is in San Juan River of Manila. Due to its excessive pollution, the government prohibits nearby settlers to raise marine life as to avoid various diseases. It was declared the "dead river" since 2003. (Courtesy of the video: GMA Public Affairs via Youtube)
Reference:https://en.wikipedia.org/wiki/Water_pollution
------------------------------------------------------------------
All rights reserved 2019. No part of this article
may be reproduced without special credits
in writing from the publishers of the given references above.