Chromium is discovered in 18th century by the analysis of Siberian ores. The two products chromium and lead will obtained. The oxides of chromium is discovered in 1797.

The chromium oxide heated at high temperature of 20 degree centigrade as a result chromium metal is formed. There are so many ores of chromium in which one ore is chromite. This chromite is black and brownish black in color.
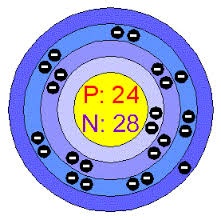
Chromium is extracted by different methods. The first method is that chromium metal is extracted from the chromite ore in which chromite ore react with sulfuric acid to farm chromium metal. Chromium is also extracted by the electrolytic process and chrome acid process.
There are so many properties of chromium in which it is steel grey metal. The density of chromium is 7.19 at a temperature of 20 degree centigrade. The melting point of chromium is 1856 .9 degree centigrade and boiling point is 2671.9 degree centigrade.

It is hard metal and coressive in nature. Chromium is used in the manufacturing of chromium wires which are used in different laboratory process. It is used in the manufacturing of steel & alloys. Chromium is used as a catalyst in the preparation of different products.

Chromium is used in manufacturing of glass to give green color. Chromium is used in the textile industries and aircraft industry. Chromium is also used has oxidizing agent in the laboratory. In our daily life, it is used for the coating of different materials.



