ENERGY BAND THEORY
Electrons of an isolated atom are bound to the nucleus, and can only have distinct energy level.However, when a large number of atoms,you can say N, are brought closer to one another to form a solid, each energy level of the isolated atom split into N sub-levels,called states, under the action of the forces exerted by the other atoms in the solid. These permissible energy states are discrete but so closely spaced that they appear to form a continuous energy band. In between two consecutive permissible energy bands, there is a range of energy states which cannot be occupied by electrons. These are called forbidden energy states, and its range is termed as forbidden energy gap.
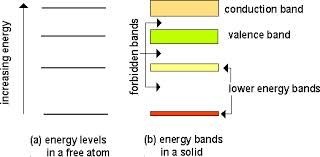
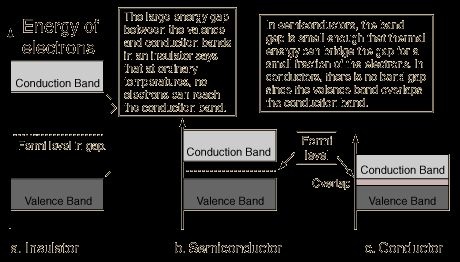
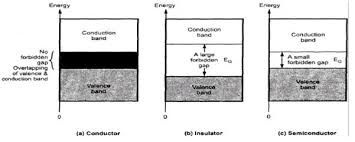
The electrons in the outer most shell of an atom are called valence electrons and the energy band occupying these electrons is known ass valence band. It is obviously the highest occupied band. It may be either completely filled or partially filled with electrons and can never be empty. The band above the valance band is called conduction band. In conduction band, electrons moves freely and conduct electric current through solids. That is why the electrons occupying this band are known as conductive electrons or free electrons.Any electrons leaving the band is accommodated by this band. It may be either empty or partially filled with electrons. The band below the valance band are normally completely filled and as such play no part in the conduction process. Thus, while discussing the electrical conductivity we will consider only the valance and conduction band.