Monosaccharides are simple sugars; they cannot be broken down into other sugars. They are categorized according to the number of carbons they contain.
Monosaccharide, also called simple sugar, any of the basic compounds that serve as the building blocks of carbohydrates. Monosaccharides are polyhydroxy aldehydes or ketones; that is, they are molecules with more than one hydroxyl group (―OH), and a carbonyl group (C=O) either at the terminal carbon atom (aldose) or at the second carbon atom (ketose). The carbonyl group combines in aqueous solution with one hydroxyl group to form a cyclic compound (hemi-acetal or hemi-ketal). The resulting monosaccharide is a crystalline water-soluble solid.
Monosaccharides are classified by the number of carbon atoms in the molecule; dioses have two, trioses have three, tetroses four, pentoses five, hexoses six, and heptoses seven. Most contain five or six. The most-important pentoses include xylose, found combined as xylan in woody materials; arabinose from coniferous trees; ribose, a component of ribonucleic acids (RNA) and several vitamins; and deoxyribose, a component of deoxyribonucleic acid (DNA). Among the most-important aldohexoses are glucose, mannose, and galactose; fructose is a ketohexose.
Source: https://en.wikipedia.org/wiki/Monosaccharide
Trioses
A triose is a three-carbon simple sugar. Trioses are formed during the metabolic breakdown of hexoses in muscle metabolism. An example of triose is glyceraldehyde (glycerose).
Courtesy of the video: Tutorialdvds via Youtube
Pentoses
Pentoses are five carbon-sugar molecules. The most important of these are ribose and deoxyribose, which are found in nucleic acids. Ribose forms part of ribonucleic acid (RNA), and deoxyribose from parts of deoxyribonucleic (DNA). Both DNA and RNA are both components of every cell nucleus and cytoplasm. The prefix de-means without, so deoxy- means without oxygen. Note that deoxyribose has one less oxygen atom than does ribose.
Other pentoses of psychologic importance include D-ribulose, which is an intermediary in the pentose phosphate shunt; D-lyxose, which is found in heart muscle; and D-xylose and D- arabinose, which are components of glycoproteins.
Hexoses
The Hexoses, the six-carbon sugars, are the most common of all carbohydrates. Of the several hexoses, the most important as far as the human body is concerned are glucose, galactose, and fructose.
Glucose
Medically, when the word glucose is mentioned, the D-isomers is meant because that is biologically active isomer. Likewise, for the other hexoses, the D-isomer is commonly called by name only, without the prefix D.
The other functions of monosaccharides includes glucose, known commonly as dextrose or grape sugar. It is a white crystalline solid that is soluble in water and insoluble in most organic liquids. It is found, along with fructose, in many fruit juices. It can be prepared by the hydrolysis of sucrose, a disaccharide, or by the hydrolysis of starch, a polysaccharide.
Glucose is found in the urine of patients suffering from diabetes mellitus and is an indication of this disease. The presence of glucose in the urine is called glycosuria. Glucose may also show up in the urine during extreme excitement (emotional glycosuria), after ingestion of large amounts of sugar (alimentary glucosuria), or because of the other factors.
Galactose
Galactose is an isomer of glucose and aldohexose. Glucose and galactose differ from each other only in the configuration of the hand OH about a single carbon atom. The sugars that differ only in configuration about a single carbon atom are called epimers. D- galactose is converted to D- Glucose in the liver by a specific enzyme called epimerase. Galactose is present in some glycoproteins and glycolipids.
Galactosemia, a severe inherited disease, results in the inability of the infants to metabolize galactose because of a deficiency of either the enzyme galactose 1-phosphate uridyl transferase or the enzyme galactokinase. The galactose concentration increases in the blood and urine (galactoseria).
Intravenous Infusion of Glucose
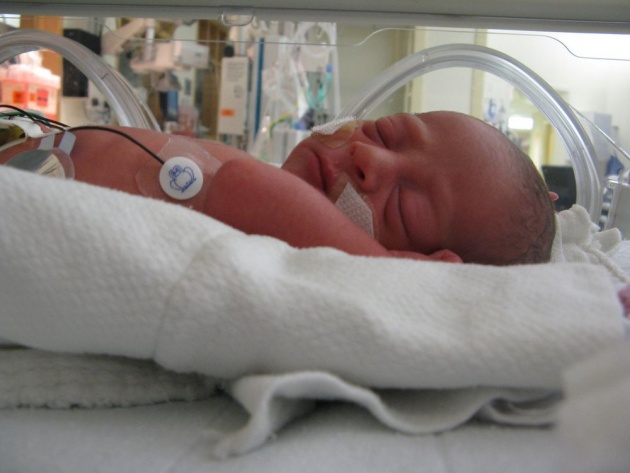
PLATE 1
A nurse looks after a premature baby infant in a pediatric intensive care unit. The plexiglasss incubator provides the infant with the warm, stable environment it needs.
Source of image: https://www.everytinything.com/nicu-articles/why-isnt-nicu-baby-dressed
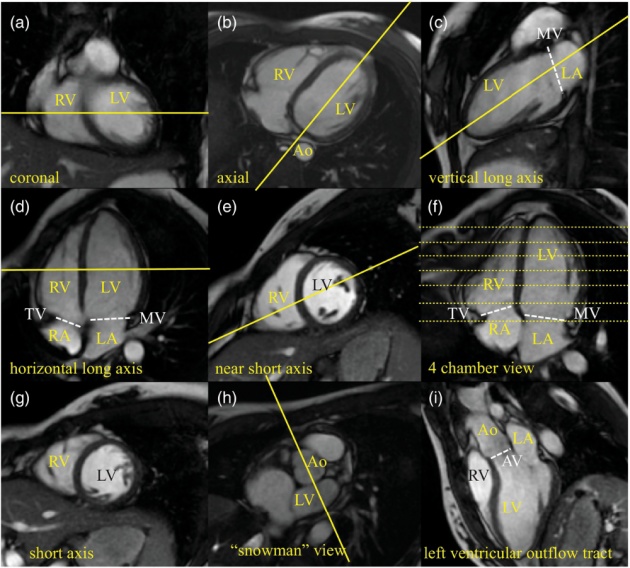
PLATE 2
Nine slices of cine display of a cardiac cycle, color enhanced to show direction and velocity of blood flow: red indicates upward flow and blue downward. Green indicates a lack distinct directional flow. In addition to quantitative flow management, the MRI technique used in this study helps asses wall motion and valve function. The blue patches show blood flow from the left atrium to the left ventricle; red patches show blood flow from the left ventricle to the aorta.
Source of image: https://www.cambridge.org/core/books/mri-from-picture-to-proton/heart-to-heart-discussion-cardiac-mri/EC77BBF504309BC71732BCBCFC7E53C7
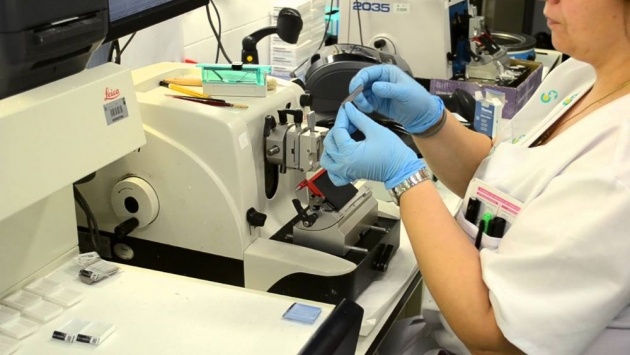
PLATE 3
A histologist examines a thin slice of tissue for the presence of cancer. Modern pathology laboratories are well-equipped to diagnose disorders found in biopsied tissue and rapidly report the results to the attending physician by telephone or fax.
Source of image: https://www.youtube.com/watch?v=3PBoHU_pidQ

PLATE 4
Laboratory technician uses a pipette to deliver specified volumes of suspended cells to an incubation heater. Such pipettes are ideal for experimental protocols that demand high accuracy and precision: pipettes can reliably dispense volumes as small as a microliter (1/1,000,000th of a liter)!
Source of image: http://www.yugopolis.ru/news/rossijskie-uchenye-razrabotali-lekarstvo-protiv-gepatita-v-113695
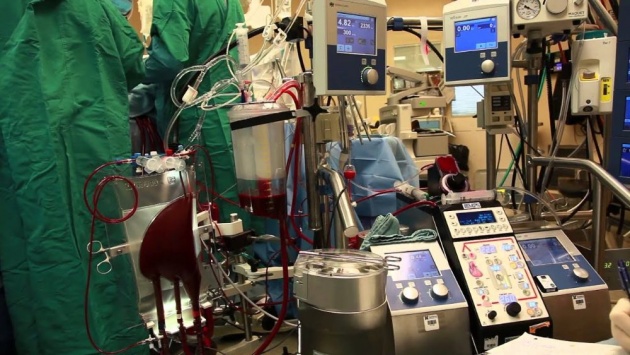
PLATE 5
The heart and lung machine is used often in thoracic surgery. The patient's blood is shunted to the machine (bypassing the heart and lungs), which oxygenates it and pumps it through the patient, hence its name.
Source of image: https://www.youtube.com/watch?v=68A5yg11DHU
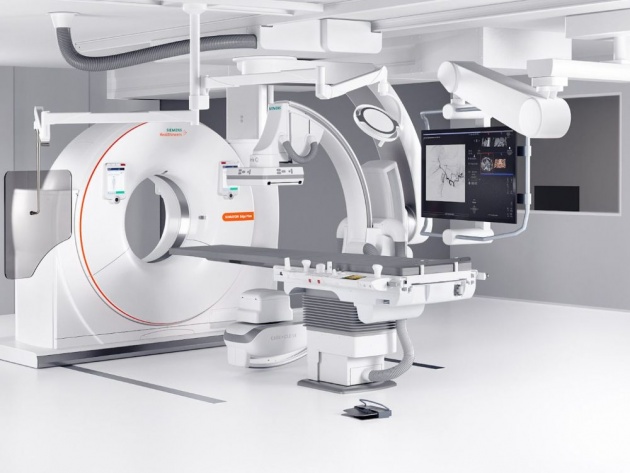
PLATE 6
Aniography is a diagnostic test used to spot potential structural problems in the blood vessels. In this procedure, an x-ray- opaque dye is injected into the circulatory system and its flow is monitored by the continuous x-ray examination. The dye outlines the blood vessels and reveals structural defects that may be present.
Source of image: https://www.healthcare.siemens.co.in/angio
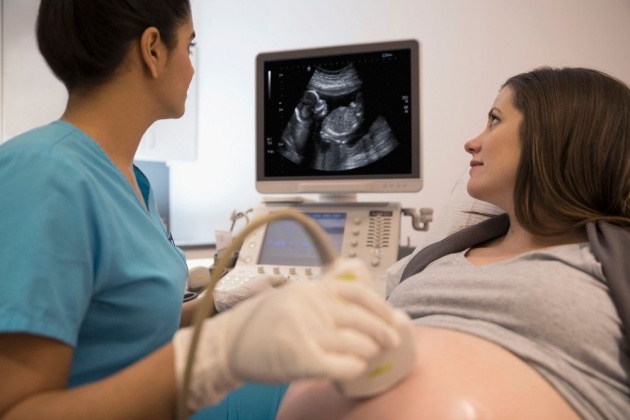
PLATE 7
A woman recieves a sonogram in a third trimester of her pregnancy. Also known as ultrasound, sonograms provide a convenient method of monitoring fetal health in utero.
Source of image: https://www.verywellfamily.com/understand-early-pregnancy-ultrasound-results-2371367

PLATE 8
Pulmonary function is measured as an individual is subjected to physical stress. Such tests quantify the performance of the lungs and readily discriminate between normal and substandard function.
Source of image: https://mft.nhs.uk/wythenshawe/services/respiratory-and-allergy/lung-function/

PLATE 9
A unit of blood is removed from the cold storage prior to use in surgery. Each unit is marked according to blood type for ease of identification. Freshly collected units of blood are tested and then stored until needed for a future operation. The need for blood is great; donated units are frequently used within a matter of days.
Source of image: http://ddnews.gov.in/health/stored-blood-may-be-unsafe-severely-injured-patients-study

PLATE 10
A Geiger counter is used to evaluate thryroid functioning. It detects radiation emitted by a perviously injected radiolabel. The distribution of the substance within the thyroid reflects the overall condition of the gland. In the picture, banana is experimented to sip its radiation.
Source of image: https://www.youtube.com/watch?v=X_XVRA5nD6Mctose
Fructose
Fructose is a ketohexose. It can be presented ass straight chain or ass ring compound. The ring structure is predominant. It is often called levulose, or fruits sugar. It occurs naturally in fruit juices and honey. It can be prepared by the hydrolysis of insulin, not a monosaccharide but a polysaccharide found in Jerusalem artichokes. Fructose is the most soluble and also the sweetest of all sugars, being 75 percent sweeter than glucose.
Fructosemia, fructose intolerance, is an inherited disease due to a deficiency of the enzyme fructose 1- phosphate aldolase. An infant suffering from the disease experiences hypoglycemia, vomiting, and severe malnutrition. Such a condition is treated by placing the infant on a low-fructose diet.
Reactions of Hexoses
Hexoses, which are either aldoses or ketoses, show reducing properties. This reducing property is the basis of the test of sugar in the urine and in the blood.
Laboratory tests for the presence of glucose in the urine using Benedict's solution of Fehling's solution, both of which contain copper (II) complex ion. Clini-test tablets, which also contain a copper (II) complex, give a rapid quantitative measurement of the concentration of the glucose present. It the blue liquid turns green, a trace of sugar (glucose) is present.
Glucose does not normally appear in the urine for an extended period of time. Its persistence present indicates that something is wrong with the metabolism of carbohydrates, such as diabetes mellitus.
- Oxidation. An aldose contains an aldehyde group, as well as several- OH groups. If the aldehyde end of the molecule oxidized, the product is named an -onic acid. When the aldehyde end of glucose oxidixed, the product is called galactonic acid. If the alcohol at the end opposite the aldehyde (the other end of the molecule) is oxidized, the product is called a -uronic acid. The oxidation of the alcohol end of glucose yields glucuronic acid. Glucunoric acid is a minor product of glucose metabolism. It is also a part of the heparin molecule.
Likewise, if the alcohol end of galactose is oxidized, galactonuric acid is formed. If both ends of the glucose molecule are oxidized at the same time, the product is called saccharic acid.
- Reduction.The aldohexoses can be reduced to alcohols. When glucose is reduced, sorbitol is formed. Sorbitol accumulation in the eye is the major factor in the formation of cataracts due to diabetes.
Reduction of galactose yields dulcitol and reduction of fructose yields a mixture of mannitol and sorbitol.
Mannitol is now used in the treatment of malignant brain tumors. Chemotherapeutic are unable to cross the blood-brain barrier, which is the brain's natural defense mechanism. The blood-brain barrier allows substances such as glucose, alcohol, and hallucinogenic agents to pass through but inhibit the entry of bacteria and toxic substances, including chemotherapeutic drugs. However, a high concentration of mannitol can be injected directly into the brain's main arteries, temporarily shrinking the cells that line those blood vessels. This shrinkage lowers the blood-brain barrier for approximately 30 minutes and allows the chemotherapeutic agents to be administered intravenously.
Fructose will also ferment; galactose will not readily ferment. Pentoses do not ferment in the presence of yeast.
- Formation of Phosphate Esters. Phosphate esters such as D-glyceraldehyde 3- phosphate and dihydroxyacetone phosphate are triose phosphate esters involved in the glycolysis.
Amino Sugars
Amino sugars (hexosamines) contain an amino group in place of an- OH group. Three amino sugars have been found in nature. Erythromycin and Carbomycin are examples of antibiotics that contain amino sugars.
Via nucleophilic displacement
Nucleophilic displacement can be an effective strategy for the synthesis of amino sugars,[4] however success strongly depends upon the nature of nucleophile, the type of leaving group and site of displacements on sugar rings. One aspect of this problem is that displacements at the C2 position tend to be slow as it is adjacent to the anomeric centre; this is particularly true for glycosides with axially-oriented aglycones.
Protein Sugar Interactions
When plasma glucose concentration is elevated over a period of time, normal hemoglobin covalently binds to glucose. The amount of glucosylated hemoglobin in the blood is used as a measure of the effectiveness of blood glucose control in a diabetic patient because the concentration of glucosylated hemoglobin directly reflects the elevation of blood glucose over the preceding several days. (Blood glucose levels directly reflect the instantaneous glucose level.)
Courtesy of the video: Mechanisms of Medicine via Youtube
***************************
Sources:
https://education.seattlepi.com/function-monosaccharide-biology-6548.html
https://blog.ifis.org/2012/food-science-and-technology/the-role-of-monosaccharides
http://orthomolecular.org/nutrients/mono.html
***********************************************
All rights reserved. No part of this article
may be reproduced without
special credits in writing from the publishers
of the given sources above.