The reactant which controles the amount of the product in chemical reaction is called limiting reactant or the reactant which consumes (finishes) earlier and stops the reaction is called as limiting reactant. Thus limiting reactant gives the least moles of product. The reactant which is taken in large amount and remains unreacted at the end of reaction is called excess reactant.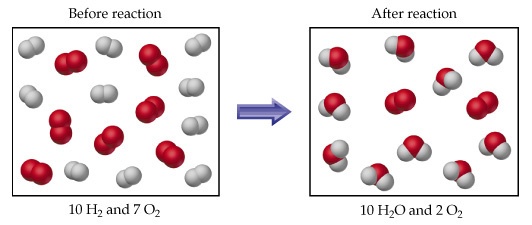
For example rection between 4 gram H_2 and 64 gram O_2 is. 2H_2 + O_(2 ) → 2H_2 O 4 grams 64 grams 4/2=2moles 64/32=2moles Now from balanced equation 2moles 〖 H〗_(2 )react with 〖 O〗_2=1mole. At the end of reaction 2 moles of H_2 will be completely consumed and reaction will be stopped. But out of two moles of oxygen,one mole will be remained unreacted. So 〖 H〗_2 is a limiting reactant and oxygen is an excess rectant. The water produced will be two moles or 36 gram.We use limiting reactant and excess reactant deliberately because They decrease loss of expensive meterials. They make the reactantion faster and faster. They make the reactions 100% complete.
Identification of limiting reactants:- We can identify the limiting reactant by three steps. Change the given mass of reactant into moles. Calculate the number of moles of products from each reactant with the help of balanced chemical equation. Identify that reactant which gives the least moles of products. It is limiting reactant. Now we take an extra interesting example of limiting reactant. We have 30 Kababs and 58 bread slices. We want to prepare Sandwiches. Each sandwich consists of one kabab and two slices. Thus we can prepare 29 sandwiches and one kabab will remain un-used. Therefore slices are limiting and kabab is excess reactant.