Nitric acid is prepared in laboratory and as well as on industries. In laboratory pure nitric acid is prepared by reacting nitrate salt with sulfuric acid by passed hot gas. In the industries, nitric acid is prepared by Ostwald process at a very high temperatures, which is 700 degree centigrade.
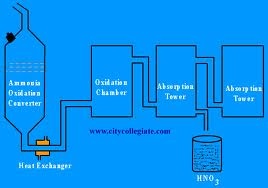
In this process, ammonia react with water to form nitric oxide and water, then this nitric oxide react with oxygen to form nitrogen dioxide.Spray of water on nitrogen dioxide, this will form nitric acid. The physical properties of nitric acid are that, it is colorless liquid and the specific gravity is 1.52 at 14 degree centigrade.
The boiling point of nitric acid is 86 degree centigrade and the smell is very choking. They irritate the sensitive tissues of human body. Nitric acid has sour taste. The chemical properties of nitric acid are that, it react with sodium hydroxide to form salt and water.
When nitric acid react with calcium carbonate to form carbon dioxide and water. When nitric acid react with magnesium to form hydrogen gas . Nitric acid is used as an oxidizing agent, when nitric acid react with copper to form nitric oxide and water.

Concentrated nitric acid react with copper to form nitrogen dioxide and water. Nitric acid is also as nitrating agent. In this case concentrated nitric acid react with methane to form nitro methane and water. Nitric acid is used as laboratory reagent.



