In Franklin's days the charge was considered to be a continuous fluid. However in these days the fluids like air or water themselves are not considered continuous but are made up of atoms and molecules. Matter is discrete. Experiments show that electrical fluid is not continuous either but it is made up of multiples of a certain elementary charge i.e any charge q that can be observed and measured directly can be written as
q = ne n = .....,-2,-1,0,1,2,.... ..............(1)
in which e, the unit of elementary charge, has the experimentally determined value equal to
e = 1.6022*10^-19 C

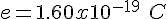
The elementary charge is one of the fundamental constants of nature.
When a physical quantity such as charge exist only in discrete packets rather in continuously varying form then the quantity is said to be quantized. We know that matter, energy and angular momentum is quantized. Charge is another important quantized quantity.
Equation (1) tells us that it is possible for example to find a particle carrying a charge of zero, + 2e, -24e etc. but it is not possible to find a particle of charge 6.24e etc.The quantum of charge is very small.
Since 1964 the physicists have used a theory of elementary particles according to which particles like neutrons and protons are considered to be made up of more fundamental particles called quarks. The quarks are assigned fractional charges of -1/3 and +2/3. Protons and neutrons are both made up of three quarks. The proton, with its net charge +e, must be composed of two +2/3 quarks and one -1/3 quark . Neutron, with its net charge equal to zero, must be composed of two -1/3 quarks and one +2/3 quark.
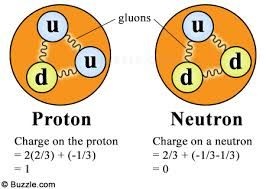
But existance of these quarks has not yet been observed and also the explanation for the failure of observation of quarks is not yet clear.