Today we talk about the procedure to find the pH of given solution . first of all we need a beaker for collecting the solution, pH paper and a solution of HCl and hydro sodium oxide.

The procedure of formation is that we take a few ml of given solution and fill it into a test tube . Now we dip strip of pH paper in this solution for finding the pH of solution. Allow the strip how to drive now compare different color of strip with standard colors.
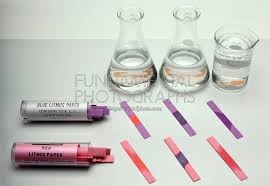
After that note the pH of solution and repeat the experiment 3 times. In this way the pH of various solution can be determined . If the color of pH paper are compared with the 1 to 5 number , thus solution are acidic.

if color of pH paper are compared with the 6 to 9 the solution are neutral and if the color of solution are compared to the 10 to 14 the solution are basic. The important thing is that what we obey the precautions of experiment.
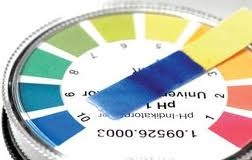
There are some important precautions of that experiment. All the glasses wares should be cleaned , dried neutral because due to these things we get the actual reading of solution. Shake this solution well before use and do not touch the filter paper with the hand.