Charles Augustin Coulomb measured electrical attraction and repulsion quantitatively and deduced the law that governs them.

Statement:-
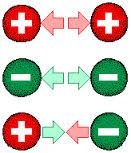
Electrostatic force of attraction or repulsion that one charge exerts on another charge is directly proportional to product of magnitude of the charges and inversely proportional to square of distance between the charges.
Mathematically:-
The force that two charges q and q' exert on each other according to Coulomb's Law is:-
F=kqq'/r.r
Here k is a constant of proportionality and its value is :-
k=8.99*10^9 Nm^2/C^2

Explanation:-
The Coulomb's Law holds for the charges whose size is much smaller than the distance between them. Generally we say that Coulomb's Law hold for point charges. The force that one charge exerts on other is directed towards the charge which exerts the force. If the two charges are of the same sign then the Coulomb's electrostatic force is repulsive and if the charges are of opposite signs then the Coulomb's electrostatic force is attractive. The electrostatic force on one charge due to a number of charges is equal to vector sum of force acted by indiviual charges.
Coulomb's Law and Newton's Law of Gravitation:-
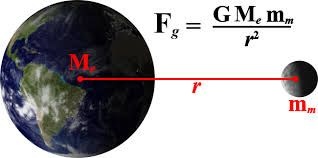
Coulomb's Law resembles Newton's inverse square law of gravitation, which was 100 years old at the time of Coulomb's experiment. Both are inverse square laws and charge q plays the same role in Coulomb's law that mass m plays in Newton's Gravitational Law. One difference between the two laws is that Gravitational force is always attractive while Coulomb's force may be attractive or repulsive. There is another major difference between the two laws that in gravitational law we define the mass in terms of force by 2nd law of motion and put it in gravitational law and get the value of G but for Coulomb's law the reverse process occurs. We define the value of constant k to have a particular value and then apply Coulomb's Law to define the unit of charge.

Significance of Coulomb's Law:-
The significance of the Coulomb's Law is that when this law was incorporated with quantum physics it correctly described the electrical forces that bind the electrons of atom to its nucleus, the forces that bind the atoms together to form molecules and the forces that bind the atoms and molecules together to form solids or liquids. Thus most of the forces around us that are not gravitational are electrical in nature.