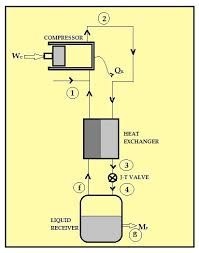
When a highly compressed gas is allowed to expand suddenly, than the cooling is produced. This cooling effect is said to be Joule Thomsen effect. A gas compressed highly having the molecules very close and strong intermoleculer forces. When the gas is allowed to expand suddenly the molecules move apart and energy is required to remove or break the intermolecular forces. This is taken from the gas itself, thus gas cools.
Linde’s method of liquefaction:-
It is used for liquefaction of all gasses except hydrogen and helium. The liquefaction of air by this method is given below:
The apparatus consists of a compressor, cooler, spiral pipe and the expansion chamber. The air is compressed about 200 atm by a compressor. Some heat is also produced in this compression. This heat is removed by cooler. Then air passes through a spiral pipe having a jet at the end.
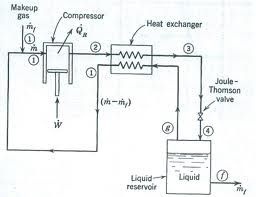
At the jet gas expands freely. The pressure decreases from 200 atm to 1 atm. Thus air is cooled. This cooled air goes up again. This