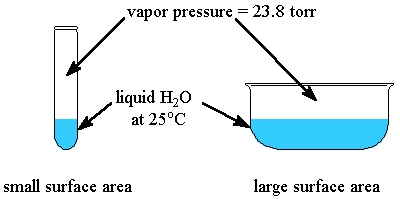
The pressure exerted by (of) the vapours in equilibrium with its liquid at given temperature is called vapour pressure of liquid. e.g vapour pressure of water is 24 torr at 〖25〗^0C. The V.P does not depend upon amount not liquid. It also does not depend on surface area of liquid.
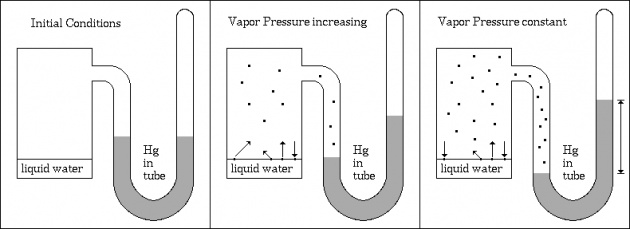
Let us suppose a liquid in a closed vessel. The vapours can not go out but they gather above the surface. These vapours can collide back with liquid surface and region it. This process is called condensation. So in a closed vessel two processes (evaporation and condensation) continue. It is shown in following figure.
After some time rate of evaporation is equal to rate of condensation. It is called state of dynamic equilibrium. Liquid ↔ Vapours At equilibrium state, the pressure exerted by the vapours is called vapour pressure.
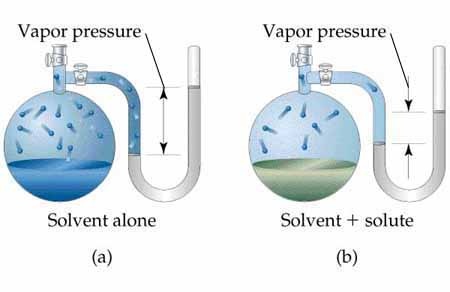
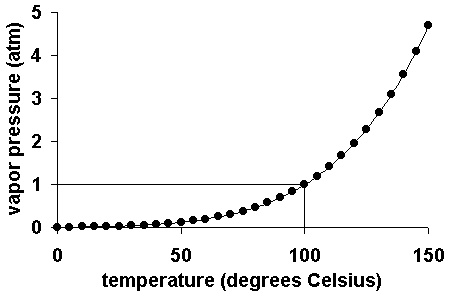
The vapour pressure depends upon nature of liquid (ii) temperature and intermolecular forces. At high temperature, the K.E of molecules is high. So intermolecular forces break down and molecules easily leave the surface. Thus V.P increases with rise of temperature.
For example when temperature of water increases from 0^0C to 〖10〗^0C, then V.P increases from 4.579 torr to 9.209 torr. Similarly when temperature of water increases from 〖90〗^0C to 〖100〗^0C, then V.P increases from 527.8 torr to 760 torr.