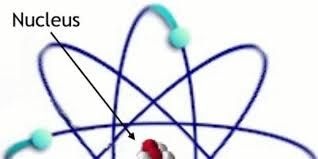
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911 as a result of Ernest Rutherford's interpretation of the 1909 Geiger–Marsden gold foil experiment. The proton–neutron model of the nucleus was proposed by Dmitry Ivanenko in 1932.[1] Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud.
The diameter of the nucleus is in the range of 1.75 fm (1.75×10−15 m) for hydrogen (the diameter of a single proton)[2] to about 15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).[citation needed]
The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.