Today we are going talk about temperature and how it can effect volume of gas.As we know that gas molecule are continuously collide each other and also with the walls of container and also each other.
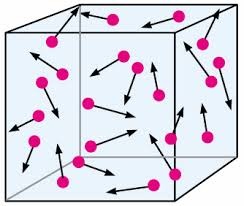
At normal temperature gas molecule are at low distance from each other, if we increase the temperature of gas molecule vibrate with larger amplitude the distance between gas molecules become higher as we increase the temperature.So increase in temperature the volume of gas become increase.

That is why we can say that temperature is directly related with volume of gas.If we increase one thing than the other thing also increase and if we decrease one thing the other thing also decrease.Mostly element are change their physical and chemical state on heat by increasing temperature and show different properties and some elements are change into gas on heat and evaporate in the air.
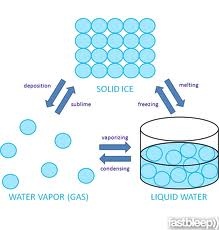
So we concluded that the change in state is depend on heat and energy that we give out side.In the laboratory we commonly use fire as a source of heat because it increase the rate of experiment and we get result in a few second.One thing is important to tell u that always be careful during any experiment because some experiment are dangerous for our body.



