In 1913 a scientist Neil Bohr presented an atomic model which is known as Bohr atomic model.
BASIS:
Bohr presented model by considering Hydrogen atom as a standard.
POSTULATES:
In order to overcome the short coming of Rutherford atomic model , Bohr presented the following postulated.
1. Electron revolve around around the nucleus in fixed circular paths called orbit or energy level or shell.
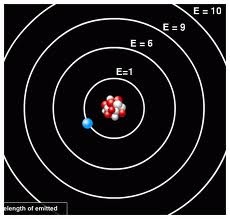
2. Energy of an orbit is fixed .It mean that when an electron revolve in a particular orbir it does not absorb or release energy.
3. An electron absorb energy when it moves from lover to higher orbit and release energy when jumps from higher to lower orbit in the form of electromagnetic waves of frequency “f”.
E=hf where h is planks constant and “f” is frequency.
Or
Energy release=energy of higher orbit – energy of lower orbit

4.Electron can stay in any one of its orbit but cannot stay between them.
5. Angular momentum of an electron in a particular orbit is integral multiple of h/2π.
mvr =n h/2π where n=1,2,3………
Success and failure:
Success;
The concept of Bohr atomic model is still valid because
.It predicts very accurately the wavelength of all the lines in the series of spectrum of Hydrogen atom.
It predicts the size of atom to be in Angstrom
(1 angstrom = 1.0 × 10-10 meters).
It provides basis for the Modern Quantum theory..
Failure:
Although Bohr model and its basic concept is still valid but there are some draw backs in this model.
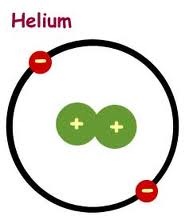
1. It is unable to explain the energy levels and spectra of complex atoms. It cannot explain even the spectrum of Helium which has only 2 electron.
2. According to Bohr atomic model electron in an orbit does not radiate energy but according to Classical Electromagnetic Theory electron being charged particle must radiate energy. SO it viollates with Classical electromagnetic Theory .
THE END



