Process Description.
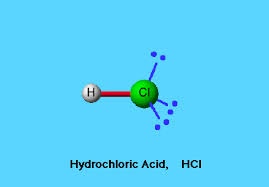
1- Production of hydrogen chloride.
In this section hydrogen chloride gas is produced by burning chlorine gas with a few percent excess hydrogen gas at high temperature 2000-2500c.these gases are available in pure form during the manufacturing of sodium hydroxide as by product .For this process a special type of furnace is used in which both gases are introduced and burnt at high temperature the reaction take place. At this stage temperature must be controlled b/w 2000 to 2500c otherwise it will be reversible reaction. The hot gas leaving the furnace at high temperature is passed through the cooler.
2- Cooling of hot gas.
The hot gas leaving top of furnace at high temperature is cooled down by passing through cooler. For this purpose different types of cooler are used which are different from each other in their design but this purpose is same cooler the hot hydrochloric acid gas. The cooling gas is now passed through absorbers.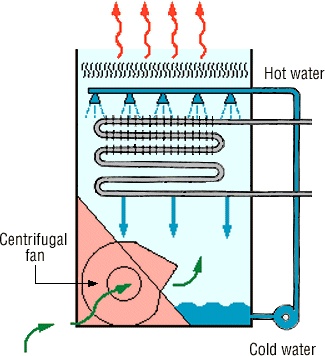
3- Absorption of hydrochloric acid gas
In this section cool hydrochloric acid gas is absorbed in water to produce by hydrochloric acid. For absorption absorbers are used where hydrochloric acid gas is introduced near the bottom and water is showered from the top of furnace hydrochloric acid is collected from the bottom of tower and stored.